NewYork-Presbyterian Begins Treating Patients with New Alzheimer’s Disease Medication
Lecanemab is the first treatment that is clinically proven to reduce the rate of cognitive decline in patients with early-stage Alzheimer’s.
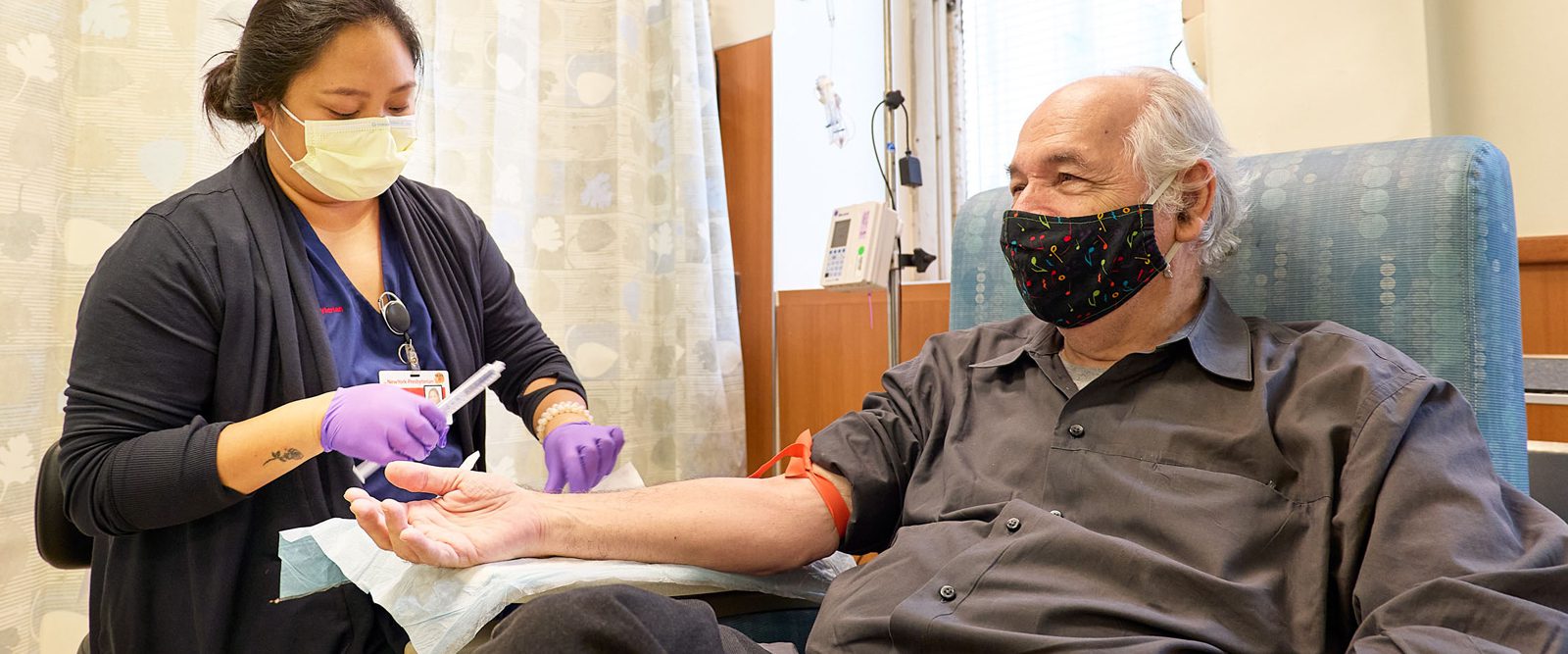

NewYork-Presbyterian began offering the new Alzheimer’s disease medication lecanemab to patients in a hospital setting. Michael Toomey, 76, the hospital’s first patient, was accompanied to his appointment by his wife of 42 years, Wendy Sharp. They said it felt good to have this new option for treatment following his recent diagnosis of Alzheimer’s disease.
“It was hard getting that diagnosis, but in some ways, it was a relief,” Michael, a former graphic designer and art director, said as the infusion nurse prepared him for his first dose of lecanemab. “We didn’t know why I was having moments of losing or forgetting things. Once we got the diagnosis, we were able to make a plan. And that plan is to fight back against this disease as best we can.”
“We understand this won’t cure Alzheimer’s, but if it’s possible it will give us more time together, it’s worth it,” added Wendy.
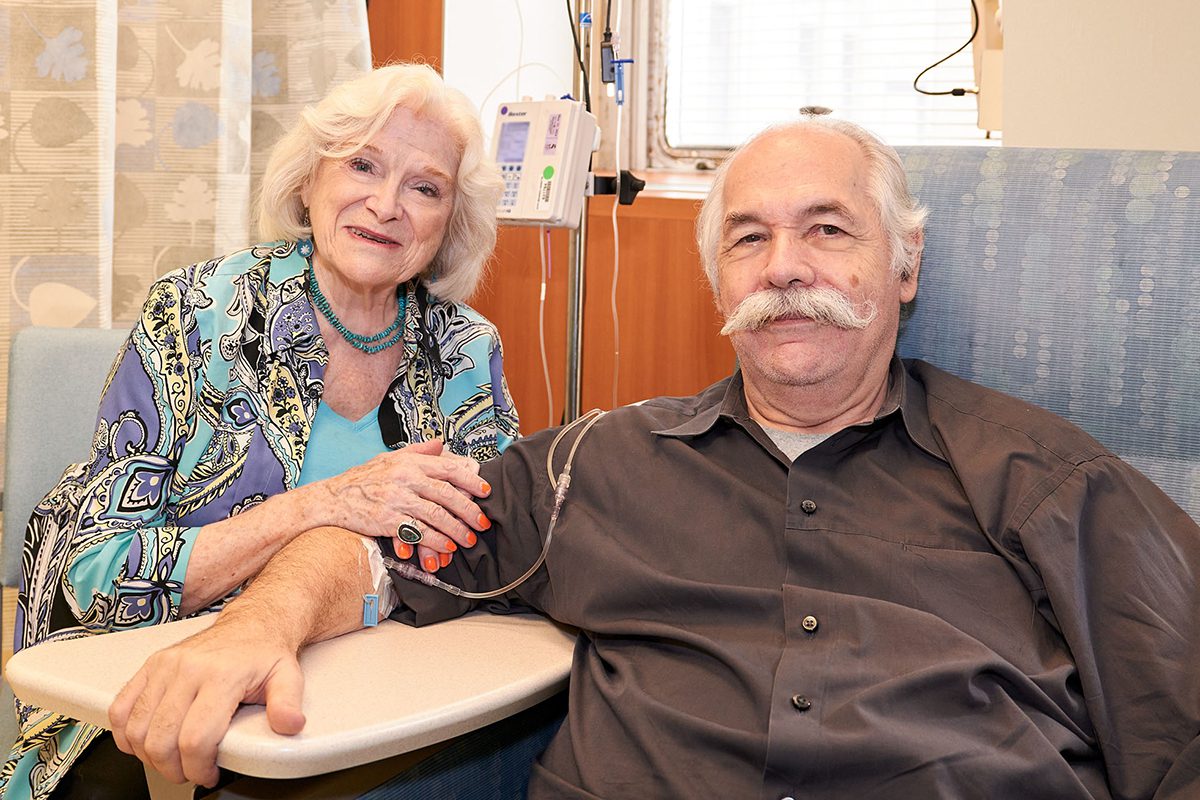
Wendy Sharp and her husband of 42 years, Michael Toomey.
Michael, who grew up in New York City, said he and Wendy, 80, first noticed his memory problems a couple of years ago when he started becoming more forgetful and losing things. At first they thought it was simply due to aging, but his memory lapses started occurring more often. About a year ago they decided it was time to consult a doctor.
Alzheimer’s disease affects approximately six million people in the U.S. and more than 30 million worldwide. The disease is associated with abnormal accumulations of the protein beta-amyloid, which forms plaques in the brain tissue, affecting memory and thinking abilities. Lecanemab, which targets the beta-amyloid protein, was designed to remove these amyloid proteins from the brain. In clinical trials, lecanemab slowed clinical decline over 18 months of treatment in people with early Alzheimer’s disease by about 27% to 37%, depending on the measure used.
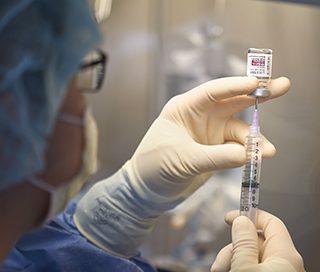
Lecanemab being prepared for infusion treatment.
Dr. Lawrence Honig, Michael’s doctor and a neurologist at NewYork-Presbyterian/Columbia University Irving Medical Center, said he is extremely pleased to be delivering anti-amyloid therapy.
“This FDA-approved therapy is a major step forward in our ability to effectively treat Alzheimer’s disease, by removing amyloid from the brain and slowing down the progression of the disease.” said Dr. Honig, who also directs the New York State Center of Excellence for Alzheimer’s Disease at Columbia. “It offers benefit not just for the patient, but also for everyone who is touched by this devastating illness. It is also accompanied by the hope that we will build on this currently available therapy, developing additional effective therapies through research here at NewYork-Presbyterian and other centers around the world.”
Additional Resources
Learn more about the Alzheimer’s Disease services available at NewYork-Presbyterian.

