What to Know About New Alzheimer’s Disease Treatments
Lecanemab and donanemab, antibody treatments administered intravenously, are new therapies clinically proven to slow the rate of cognitive decline.
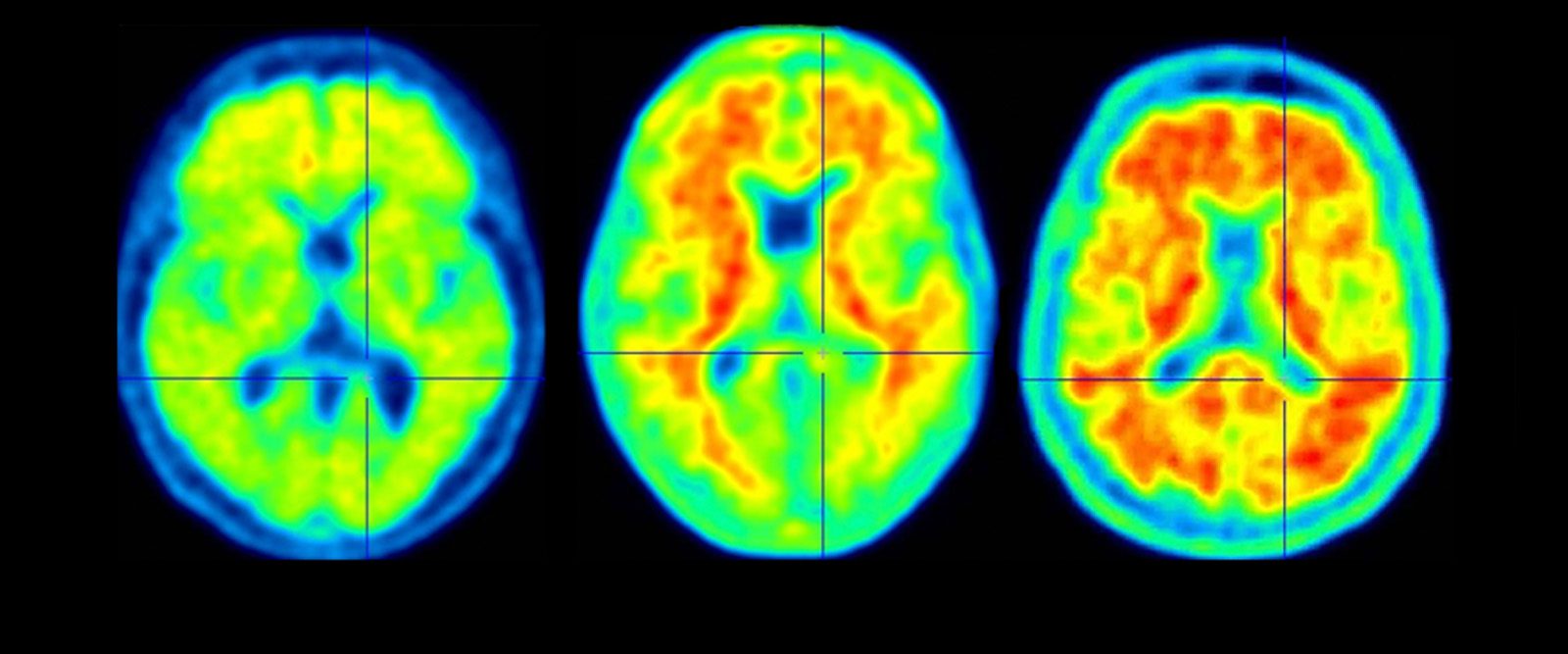
A scan of a healthy brain (left) and scans of brains with accumulations of amyloid (in red), a protein that increases the risk of Alzheimer’s disease.
Donanemab, a therapy that targets amyloid proteins thought to cause Alzheimer’s disease, was recently granted traditional approval by the Food and Drug Administration (FDA). Donanemab will be sold under the name Kisunla, and marks a continued breakthrough in the treatment of Alzheimer’s disease, which affects approximately 6 million people in the U.S.
Donanemab joins lecanemab — marketed as Leqembi — as one of two drugs that remove Alzheimer’s protein from the brain and clinically slow Alzheimer’s disease. Lecanemab received traditional approval in July 2023. (Aducanumab was the first anti-amyloid antibody therapy approved by the FDA, but has since been discontinued.)
Alzheimer’s is associated with abnormal accumulations of the protein beta-amyloid, which forms plaques in the brain tissue, affecting memory and thinking abilities. “The anti-amyloid antibody therapies target forms of the beta-amyloid protein in the brain of people with early-stage Alzheimer’s disease,” says Dr. Lawrence S. Honig, a neurologist at NewYork-Presbyterian/Columbia University Irving Medical Center. “Clinical trials for both fully approved drugs have found dramatic evidence of removal of amyloid proteins in the brain by the intravenous version of the treatments and have clinically proved that these drugs slow down progression of the disease.”
Since September 2023, neurologists at NewYork-Presbyterian have been administering lecanemab to patients at the Columbia, Cornell, and other NYP locations. Every two weeks, patients receive the therapy intravenously.
Health Matters spoke with Dr. Honig to understand how the treatments work and their risks.

Dr. Lawrence Honig
How do lecanemab and donanemab work? What promise do these therapies hold and how do they differ?
Dr. Honig: Lecanemab is a monoclonal antibody that binds amyloid and was designed to remove amyloid from the brain. It has long been suggested that removing the protein might slow the progression of Alzheimer’s disease. Donanemab is another drug of the same broad class. Like lecanemab, it removes beta amyloid and slows disease progression.
There are also differences between both treatments, including in side effects, drug administration, and possibly in length of treatment. It is not certain that patients on donanemab will continue to benefit from the medication once there is substantial clearance of the amyloid proteins from the brain. Donanemab infusions require dose escalation, with a different starting dose for the first few months. Doses for donanemab are administered to patients every four weeks, while doses for lecanemab are given every two weeks.
Do lecanemab and donanemab reverse problems linked to Alzheimer’s disease, its signs, and its symptoms? They do not unfortunately reverse the symptoms. Do they slow them down? Yes, they do slow Alzheimer’s disease as measured in a variety of ways, including by reducing amyloid levels. And these are the first medications clinically proven to do so.
What did the clinical trials for both treatments find?
In lecanemab’s phase 3 clinical trial, there were 1,795 participants between 50 to 90 years of age who were in the early stages of Alzheimer’s disease. Every two weeks, they received the treatment by intravenous infusion. In clinical trials, phase 3 is the last testing phase before a drug is submitted for regulatory approval and released in the market. Results of the clinical trial, which were published in the New England Journal of Medicine, showed that it slowed decline for people with early Alzheimer’s disease by about 27% to 37%, depending on the clinical test measure. After 18 months of treatment, the mean amyloid level in most treated patients was below the threshold for amyloid positivity, meaning that a radiologist would not even know the patient had Alzheimer’s disease.
In donanemab’s phase 3 clinical trial, which were published in a study in the Journal of the American Medical Association, treatment with the drug was associated with dramatic amyloid removal from the brain, as well as slowing of progression of the disease for patients in the early stages of Alzheimer’s. There were 1,736 participants between 60 to 85 years who were in early stages of disease. The trial found that in the full group of patients, donanemab treatment slowed decline in memory and thinking, and functional measures by 20% to 29%, depending on the clinical scale.
The main risks of both medications include brain swelling and brain bleeding, which, at some levels, participants in studies of both drugs experienced.
What do we know about subcutaneous or injectable lecanemab?
A subcutaneous version of lecanemab – consisting of weekly injections under the skin – is showing promising results, according to data recently shared by Eisai, one of the manufacturers of the therapy, at the 16th annual Clinical Trials on Alzheimer’s Disease conference. In preliminary results of a substudy on subcutaneous lecanemab, patients who received weekly injections of the therapy showed comparable amyloid plaque removal and similar side effects to those who got lecanemab intravenously every two weeks. The subcutaneous substudy included 72 patients who received lecanemab for the first time as the subcutaneous formulation, and 322 patients who originally received lecanemab intravenously in the phase 3 clinical trial, but then received subcutaneous administration in this substudy.
Patients showed similar concentrations of drug by injection under the skin once a week by intravenous infusion every two weeks. In some ways, the delivery looked more favorable. Instead of the ups and downs seen with the intravenous infusion, with high concentrations of drug and then lower concentrations as time goes on, it seems like the level of the drug was more stable and consistent with the subcutaneous injection.
Use of subcutaneous lecanemab is still being studied, and the data is being submitted on a rolling basis to the FDA for later consideration of approval. For lecanemab, data has also been submitted to the FDA for possible maintenance dosing at every four weeks, after initial dosing every two weeks that is currently approved for the first 18 months of treatment.
Are lecanemab and donanemab covered by insurance?
The Centers for Medicare & Medicaid Services (CMS) announced the broad availability of coverage for medications directed against amyloid for the treatment of Alzheimer’s disease that have received full FDA approval. There are some restrictions for these medications, including that they are only indicated for people who have early Alzheimer’s disease, namely either mild cognitive impairment or mild dementia due to this disease, and have confirmation of the presence of beta-amyloid accumulation.
CMS said that the therapies will be covered as long as information on patients is entered in one of the privacy-protected registries of treated patients. These registries are databases designed to collect information about how the drug works in the real world.
Lawrence S. Honig, M.D., Ph.D., is a neurologist at NewYork-Presbyterian/Columbia University Irving Medical Center and professor of neurology at the Columbia University Vagelos College of Physicians and Surgeons, specifically in its Department of Neurology, the Taub Institute for Research on Alzheimer’s Disease and the Aging Brain, and the Gertrude H. Sergievsky Center, where he directs the New York State funded Center of Excellence for Alzheimer’s Disease. Dr. Honig’s clinical specializations focus on Alzheimer’s disease, Lewy body dementia, frontotemporal dementia, progressive supranuclear palsy, Creutzfeldt-Jakob disease, immune-mediated encephalitis, and other disorders of nervous system aging and degeneration.
Dr. Honig was an investigator for lecanemab’s CLARITY AD clinical trial carried out at NewYork-Presbyterian/Columbia and has been an investigator and received research funding from Biogen Eisai, and Lilly for other drug studies. He also has consulted for Eisai, the pharmaceutical company developing lecanemab, as well as for Biogen, Cortexyme, Genentech, Lilly, Prevail, and Roche pharmaceutical companies.
