COVID Vaccine and Allergies: What You Should Know
An allergist-immunologist answers top questions about what people with allergies need to know about the COVID-19 vaccines.
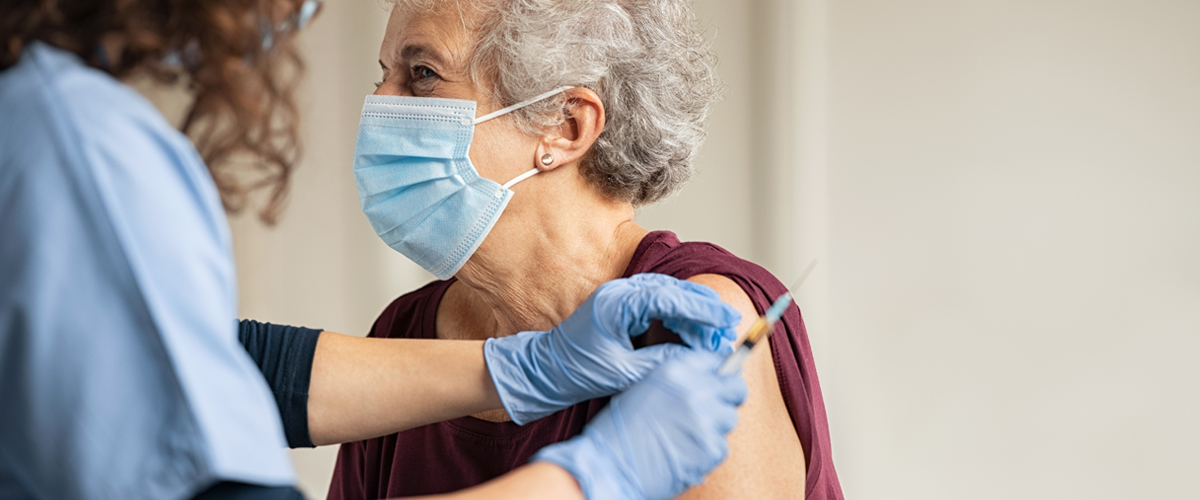
Millions of doses of the coronavirus vaccines are rolling out across the country. As some rare cases of severe allergic reactions have been reported, people with allergies may wonder what it means for them and whether they should still receive a COVID vaccine.
“These cases of a severe allergic reaction are very rare,” says Dr. Jordan Scott Orange, physician-in-chief at NewYork-Presbyterian Morgan Stanley Children’s Hospital and chair of pediatrics at Columbia University Vagelos College of Physicians and Surgeons. “The vast majority of people with a history of allergies can receive this vaccine safely, but it’s important to be educated about these instances.”

Dr. Jordan S. Orange
Dr. Orange, an allergist-immunologist, says the rare reactions should not deter the general public, including those with allergies, from getting the coronavirus vaccination.
“This is really a wonderful moment for us all, as this presents the ability to save so many lives, for us to truly flatten the curve to protect our patients, to protect ourselves, and protect our families,” says Dr. Orange.
Here, Dr. Orange tells Health Matters what people should know about the COVID-19 vaccines and allergies.
Can someone who has other kinds of severe allergies, such as an allergy to nuts or animals or to a plant, get the vaccine?
Many individuals who have had a history of life-threatening reactions to peanuts, tree nuts, specific foods, or bee stings have safely received this vaccine. Those kinds of allergies are not contraindications for getting the vaccine. People with these common allergies can safely get vaccinated. Many have already.
What if you have a family history of a severe allergic reaction?
A family history of an allergic reaction, even if it’s a family history of an allergic reaction to a vaccine, does not create a risk for this vaccine. Those individuals can safely receive, and have received, the vaccines. Let’s keep in mind that millions of people at this point have safely received the vaccines.
What is a severe reaction, when does it occur, and how is it treated?
A severe reaction, or anaphylaxis, is an immediate allergic reaction. For example, if someone is allergic to nuts and they ingest a nut, their face may swell, their throat may swell, and/or they may start vomiting. Anaphylaxis, when it occurs, is treated by an epinephrine (adrenaline) injection. The Centers for Disease Control and Prevention (CDC) says an example of an allergic reaction that’s severe is when a person needs to be treated with epinephrine, such as EpiPen, or they must go to the hospital. We are not talking about common reactions to the vaccine such as soreness in the arm and fatigue. An allergic reaction typically occurs within 30 minutes after receiving the vaccine.
Who should not receive a COVID-19 vaccine?
The CDC says that people who have had a severe allergic reaction, specifically anaphylaxis, or an “immediate allergic reaction” (such as hives, swelling or wheezing within four hours) to an ingredient in either the Pfizer or Moderna COVID-19 vaccines should not get either one. Also, people who have a severe or immediate allergic reaction after the first COVID-19 shot should not get the second shot of either mRNA vaccine.
What about people who have had severe allergic reactions to other vaccines in the past?
There is a cautionary group that should consult with their allergist before receiving a COVID-19 vaccine. These are people who have had an allergic reaction to another vaccine — not the COVID vaccine but to another vaccine. Or they have a history of an allergic reaction to an injectable medicine, as some of the liquid components of injectable medicines may have similarities to what is in a vaccine. So, people who fall into either of those categories should consult with their allergist beforehand.
The vast majority of people with a history of allergies can receive this vaccine safely, but it’s important to be educated about these [rare cases of severe allergic reactions].
Dr. Jordan S. Orange
Why are people advised to wait for a period of time after receiving the vaccine?
The COVID vaccines require a 15-minute observation period to watch for any kind of reaction, but that’s not specific to these vaccines. The standard practice from the Advisory Committee on Immunization Practices, which is the organization that advises the CDC on vaccination practices, recommends a 15-minute observation period as a best practice for vaccination in order to be monitored. That applies to any vaccine we receive, and the COVID-19 vaccines are no different. That’s just good practice, and it applies to everyone.
For those who have a history of anaphylaxis or an immediate reaction to a vaccine or an injectable therapy, or have another personal history of severe, life-threatening allergies, they should be observed for 30 minutes. So, just a little bit of an extended observation period for those individuals.
Some experts are pointing to a component called PEG as a possible cause. What is it?
While the cause of vaccine anaphylaxis has not yet been proved, some think that it may be something called PEG. PEG, or polyethylene glycol, is a stabilizer found in many medicines such as Tylenol gels, ultrasound jelly, and Miralax (bowel preps and constipation medicines). Allergy to PEG is extremely rare. PEG is contained in both the Pfizer and Moderna vaccines, and since the shots contain very few ingredients, PEG seems to be a potential explanation. We will learn more soon and even get to specific diagnoses for people who have had a reaction. Consult with an allergist if you’re unsure. An allergy to PEG may be suspected in anyone who has had an immediate severe allergic reaction to certain injectable drugs that contain PEG.
Again, as this is a rapidly developing story, people are advised to check back regularly. The good news is that despite millions of doses of vaccine given, the serious (anaphylactic) reactions remain rare.
Jordan Scott Orange, M.D., Ph.D., is the physician-in-chief at NewYork-Presbyterian Morgan Stanley Children’s Hospital and chair of pediatrics at Columbia University Vagelos College of Physicians and Surgeons. An international leader in pediatric primary immunodeficiency and the immunobiology of human natural killer cells, Dr. Orange in his research combines novel disease discovery with basic cell research to translate underlying biological mechanisms of disease into clinical applications. His research has been continuously funded by the National Institutes of Health and he has published over 250 papers. Dr. Orange is a member of the American Society for Clinical Investigation and the American Pediatric Society and was a recipient of the E. Mead Johnson Award for research accomplishment in pediatrics from the Society for Pediatric Research.
