What Are the Common Side Effects of the Updated COVID-19 Vaccine?
An infectious disease expert shares what people might feel after getting the updated COVID-19 shot and what reactions to the vaccine might mean about immunity.
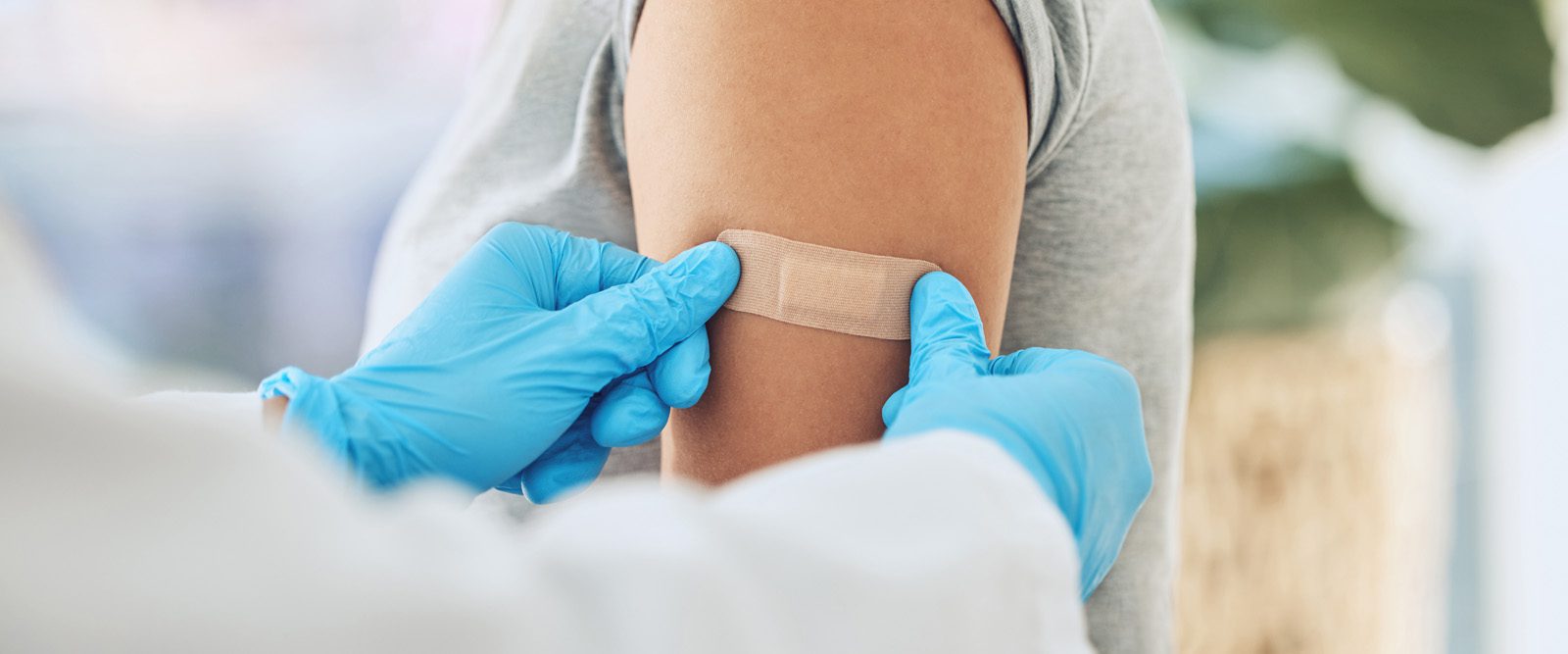
Fatigue, muscle pain, headache — if you’re feeling lousy after your most recent COVID-19 vaccine, it could be a sign your immune system is building antibodies to help protect you against future infection.
With new COVID-19 vaccines available, the updated shots are expected to better target new variants, according to experts. The Centers for Disease Control and Prevention (CDC) has recommended the updated vaccines, made by Pfizer-BioNTech and Moderna for anyone 6 months and older, and Novavax for anyone 12 and older.

Dr. Magdalena E. Sobieszczyk
“People may experience side effects from the vaccine, but they are often short-lived and outweighed by the long-term benefits,” says Dr. Magdalena Sobieszczyk, chief of the Division of Infectious Diseases at NewYork-Presbyterian/Columbia University Irving Medical Center. “Though hospitalizations and deaths have decreased, it’s important to get the updated vaccine because it can prevent serious infections, not only among the general population but also in vulnerable populations and younger individuals.”
So what does it mean if you’re feeling sick soon after receiving the shot, and how can you manage any reactions? Dr. Sobieszczyk, who is also a professor of infectious diseases in medicine at Columbia University Vagelos College of Physicians and Surgeons, shares what you can expect from the updated COVID-19 vaccine, including potential side effects, antibody levels, and more.
What’s the difference between the updated COVID-19 vaccine and those from previous years?
Dr. Sobieszczyk: This updated vaccine is not called a booster, as it’s not just boosting the existing immunity from previous or recent vaccines. It provides updated protection and helps the body build new responses against the new variants. Similar to the yearly flu vaccine, the FDA is anticipating that they will need to meet on an annual basis to understand how COVID-19 is evolving, review data on circulating strains, and advise manufacturers on which strains should be selected for the vaccine each year.
What are common side effects of the COVID-19 vaccine?
Common reactions that may appear within one to two days of receiving the vaccine include:
- Pain, swelling, or redness at the injection site
- Fatigue
- Headache
- Mild fever
- Nausea
- Muscle pain
These symptoms are normal and not dangerous.
What’s happening in the body if someone is experiencing side effects from a vaccine?
Typically, when people are experiencing symptoms after any vaccine, it could be a sign that their immune system is responding: Their antibody levels are increasing and their T cells, which help fight disease, become active.
"People may experience side effects from the vaccine, but they are often short-lived and outweighed by the long-term benefits."— Dr. Magdalena Sobieszczyk
Why do some people experience reactions to a vaccine while others don’t?
Everybody’s immune system has unique characteristics, and that can influence how people may respond to a vaccine. What people are experiencing can be multifactorial. It can be a function of what else is going on in their lives. Maybe they’re more tired or stressed, or maybe they had another recent infection that their immune system is already responding to.
Age also can play a role. In larger COVID-19 vaccine studies, people below the age of 55 tended to have more reactions like fever, fatigue, headache, and muscle aches, and that may have to do with how vigorous the immune system is. As you get older, your immune system tends to quiet down a bit.
With this current vaccine, what we are hearing is that for the vast majority on a large population level, the side effects are on par with previous doses and people aren’t feeling any worse from the updated shot.
It’s also important to remember that we are in flu season, along with other respiratory viruses circulating in addition to COVID-19. If symptoms last longer than a day or two, people may think the vaccine is the reason they’re getting sick or testing positive for COVID, but it’s likely a separate infection or they’re developing symptoms concurrently.
Do more vaccine symptoms mean more protection?
I’d caution against the idea that there is no protection if there is an absence of symptoms like pain, mild fever, or fatigue.
There are some studies that show that people have higher antibody levels when they have strong symptoms after vaccination, but the majority of people make antibodies after the vaccine. The differences that we see are not substantial enough to make us worry about lack of protection.
Should you take a pain reliever before the vaccine to prevent any symptoms?
I don’t recommend taking a pain reliever like ibuprofen or acetaminophen prior to the vaccine as a standard process, but would rather someone take one afterward if they’re feeling any pain or headache.
How can you treat COVID-19 vaccine side effects at home?
- Stay hydrated and drink lots of fluids one to two days after getting the vaccine.
- Get plenty of rest.
- For arm pain or swelling, move your arms frequently after getting the vaccine, and keep it elevated to ease discomfort.
- Take a pain reliever for headache, pain, or muscle aches.
- Apply a cool, wet washcloth or ice pack over the injection site to help relieve any pain.
When should someone see a doctor if they’re experiencing any vaccine-related symptoms?
Typically, someone can expect to experience symptoms for a day or two. But if the pain isn’t going away or it’s getting worse after that time frame, get in touch with a healthcare provider.
Allergic reactions are very rare, but if someone has trouble breathing, persistent pain or pressure in their chest after getting the vaccine, they should contact their physician.
Magdalena Sobieszczyk, M.D., is the chief of the Division of Infectious Diseases at NewYork-Presbyterian/Columbia University Irving Medical Center and is the Harold Neu professor of infectious diseases in medicine at Columbia University Vagelos College of Physicians and Surgeons. Dr. Sobieszczyk is also a clinical virologist and the principal investigator of the Columbia Collaborative Clinical Trials Unit, funded by the National Institutes of Health, which has been advancing the science of infections like SARS-CoV-2 and HIV.

