Can Gene Therapy Cure Patients with Sickle Cell Disease?
An experimental trial shows promise for patients with a life-threatening inherited blood disorder.
A team at NewYork-Presbyterian and Columbia University Irving Medical Center is pioneering a potential new treatment that may offer hope for patients with sickle cell disease (SCD), a devastating inherited blood disorder that causes anemia, infection, debilitating pain, and organ damage.
More than 100,000 Americans and millions more across the globe have this life-threatening disease, which causes red blood cells to change shape and malfunction, clogging blood vessels and preventing oxygen from moving throughout the body.
SCD is more common among people of African descent. Experts believe the genetic trait evolved because it protects people from malaria. In the United States, SCD occurs in about 1 of every 365 black or African-American births, according to the Centers for Disease Control and Prevention.
“Patients who are born with SCD, depending on the severity, have a reduced life expectancy, and so far the only way of curing this disease is through a bone marrow or stem cell transplant,” says Dr. Markus Mapara, director of the Bone Marrow Transplantation and Cell Therapy Program at NewYork-Presbyterian/Columbia University Irving Medical Center. “Unfortunately, the chances of having a matched sibling donor, which provides the best outcomes, are only 25%. So it’s important for us to find other ways to cure this disease.”
Today, one new approach is showing promise. Dr. Mapara is leading a groundbreaking clinical trial at NewYork-Presbyterian/Columbia University Irving Medical Center, which is part of a larger multicenter trial, that is using gene therapy to try to cure SCD patients. Health Matters spoke with Dr. Mapara to learn about the experimental trial that has shown encouraging results for patients.
“Advances in gene therapy are unlocking potential new treatments for this disease, and I am excited that they may offer hope and a new option for those suffering from SCD,” says Dr. Mapara.
Why is SCD a candidate for gene therapy?
Dr. Mapara: Scientists have long known that SCD is caused by only one errant gene, ranking it high on the list of diseases that could be potentially cured with gene therapy, as opposed to diseases with multiple genes affected. If you could fix that one defective gene, you could potentially cure the disease and reverse the genetic malfunction.

Dr. Markus Mapara
How is sickle cell disease currently treated?
So far, all treatments have focused on symptom relief. Outside of new gene therapy trials, the only way of treating or curing sickle cell is through a bone marrow or stem cell transplant: A healthy donor can donate stem cells (blood-forming cells) taken from the bone marrow (the soft, spongelike tissue in the center of most bones that produces the blood cells) or the bloodstream to replace the malfunctioning stem cells for a person with SCD.
Why are new treatments needed?
Transplants from a matched sibling are very safe, but unfortunately, the chances of having a sibling match are just 25%. The risks are higher with a donor who is not related by family, in terms of treatment-related complications or transplant rejection. This is why it is important for us to explore other ways to cure this disease. Gene therapy would provide a definitive treatment for SCD without relying on whether a donor is available.
What is the gene therapy treatment your team is researching?
NewYork-Presbyterian/Columbia Irving Medical Center is participating in a clinical trial sponsored by Bluebird Bio, a biotech company, to test a gene therapy for SCD. Through the trial, we are giving patients new and genetically engineered hemoglobin (the molecules in red blood cells that deliver oxygen throughout the body) through a stem cell transplant, using the patient’s own stem cells. We want to introduce healthy hemoglobin to prevent the red blood cells from malforming.
Basics of SCD
What is sickle cell disease?
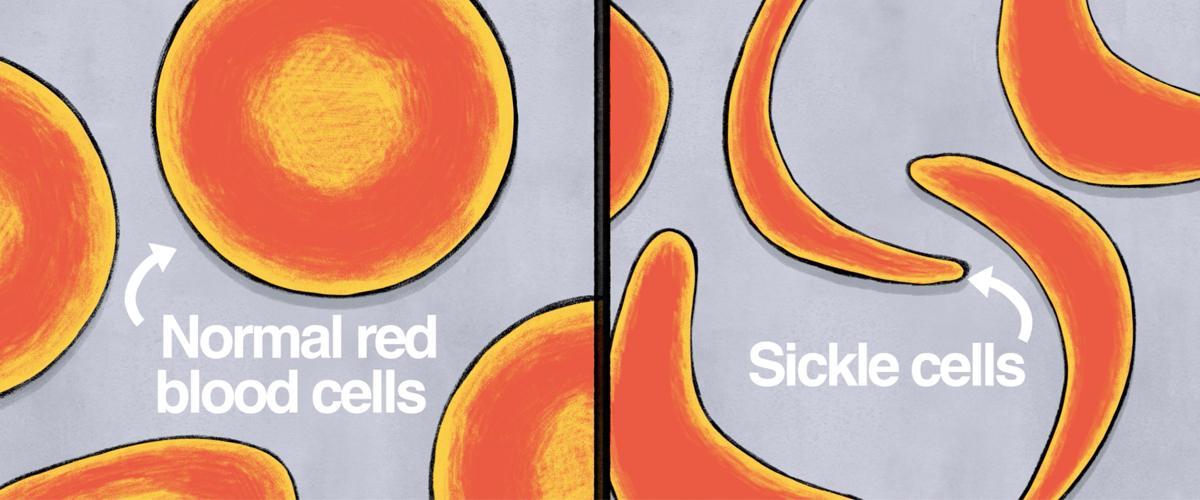
Dr. Mapara: Normal red blood cells have a round disc shape and are flexible, allowing them to move through vessels throughout the body. For those with SCD, a gene defect causes red blood cells to become shaped more like a crescent moon or a sickle (an old farm tool). These misshaped cells clog up small blood vessels, obstructing blood flow to organs and driving symptoms.
What are the symptoms of SCD?
Sickled blood cells cannot carry adequate oxygen to the body’s tissues, causing anemia, characterized by fatigue and weakness. SCD is not only painful but can also damage organs such as the lungs, kidneys, or liver. It can also lead to stroke, infections, and bone destruction. In some cases, SCD can reduce life expectancy.
When do people begin to show symptoms?
Some patients have many crises — episodes of agonizing pain — when they are children. For others, the disease remains dormant in childhood, and then as they grow older they suddenly have one crisis after the other. Generally, more complications develop as a patient grows older.
How is this treatment done?
The patient’s stem cells are removed from their blood and sent to a laboratory where they are mixed together with a new, corrected gene. The genetically engineered cells are then returned to the patient’s bloodstream, and the hope is that the stem cells with the modified DNA will start producing healthy red blood cells.
Interestingly, the way the new gene is delivered to the patient’s stem cells is through a virus. This trial involves inserting the new gene into a deactivated virus that is especially good at inserting the new gene into the stem cell.
What are the results of the trial so far?
This trial is being tested at seven sites, and Bluebird Bio has followed patients for more than three years. The data is showing promising outcomes in terms of increased healthy hemoglobin levels and reduced pain episodes.
So far, we have transplanted three patients and enrolled a total of five, with encouraging results.
Are you hopeful for the future?
The results of the trial are promising, which is exciting, as gene therapy would provide a potentially better option than a bone marrow/stem cell transplant since patients would not need to rely on finding a perfectly matched donor. I am hopeful and looking forward to seeing the long-term results.
Other experimental approaches are being explored as well, and I’m optimistic that treatments will improve for this patient population. This is a young population of patients who have suffered through childhood and adolescence, and it’s gratifying to have the possibility of giving them their lives back.
Dr. Mapara reports no financial or other conflicts of interest.
Markus Mapara, M.D., Ph.D., is the director of the Bone Marrow Transplantation and Cell Therapy Program at NewYork-Presbyterian/Columbia University Irving Medical Center and a professor of medicine at Columbia University Vagelos College of Physicians and Surgeons.
